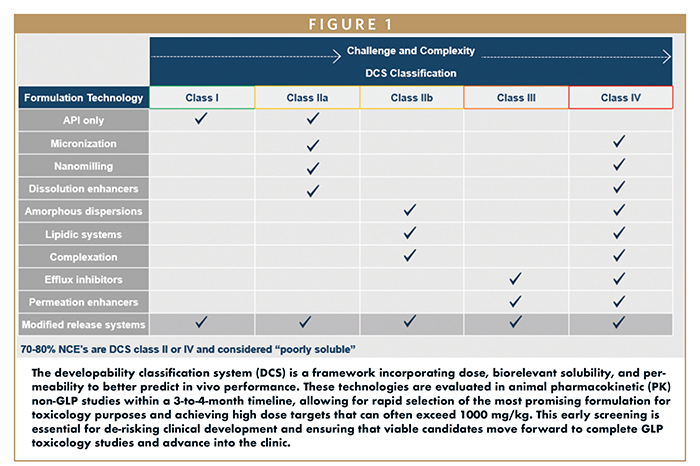
Quotient Sciences has unveiled a preclinical screening platform that significantly enhances the development of poorly soluble drugs, addressing a critical barrier in pharmaceutical R&D. With approximately 40% of marketed pharmaceuticals and nearly 90% of investigational compounds suffering from poor aqueous solubility, the implications for bioavailability and therapeutic efficacy are profound. This platform integrates advanced formulation technologies, such as amorphous solid dispersions and lipidic systems, to optimize drug solubility and absorption.
By employing a systematic, data-driven approach, Quotient Sciences enables development teams to streamline the transition from preclinical phases to first-in-human trials. This integrated service model not only reduces supply chain complexities but also allows for real-time formulation adjustments based on clinical data. The success of this platform is exemplified by a recent study on an oral therapy for amyotrophic lateral sclerosis, where an optimized formulation demonstrated superior pharmacokinetics. As the industry grapples with high drug development failure rates, Quotient’s innovative strategies could redefine the landscape of drug formulation, ultimately enhancing patient outcomes and commercial viability.
Start your 7-day trial and see what the database can do →