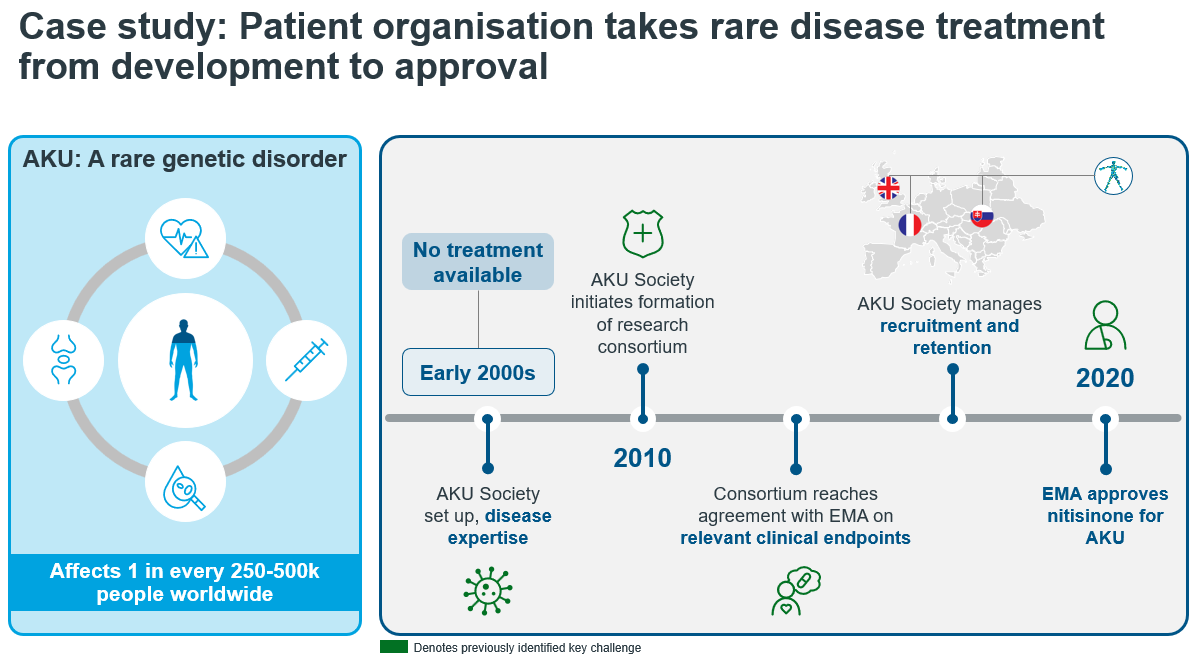
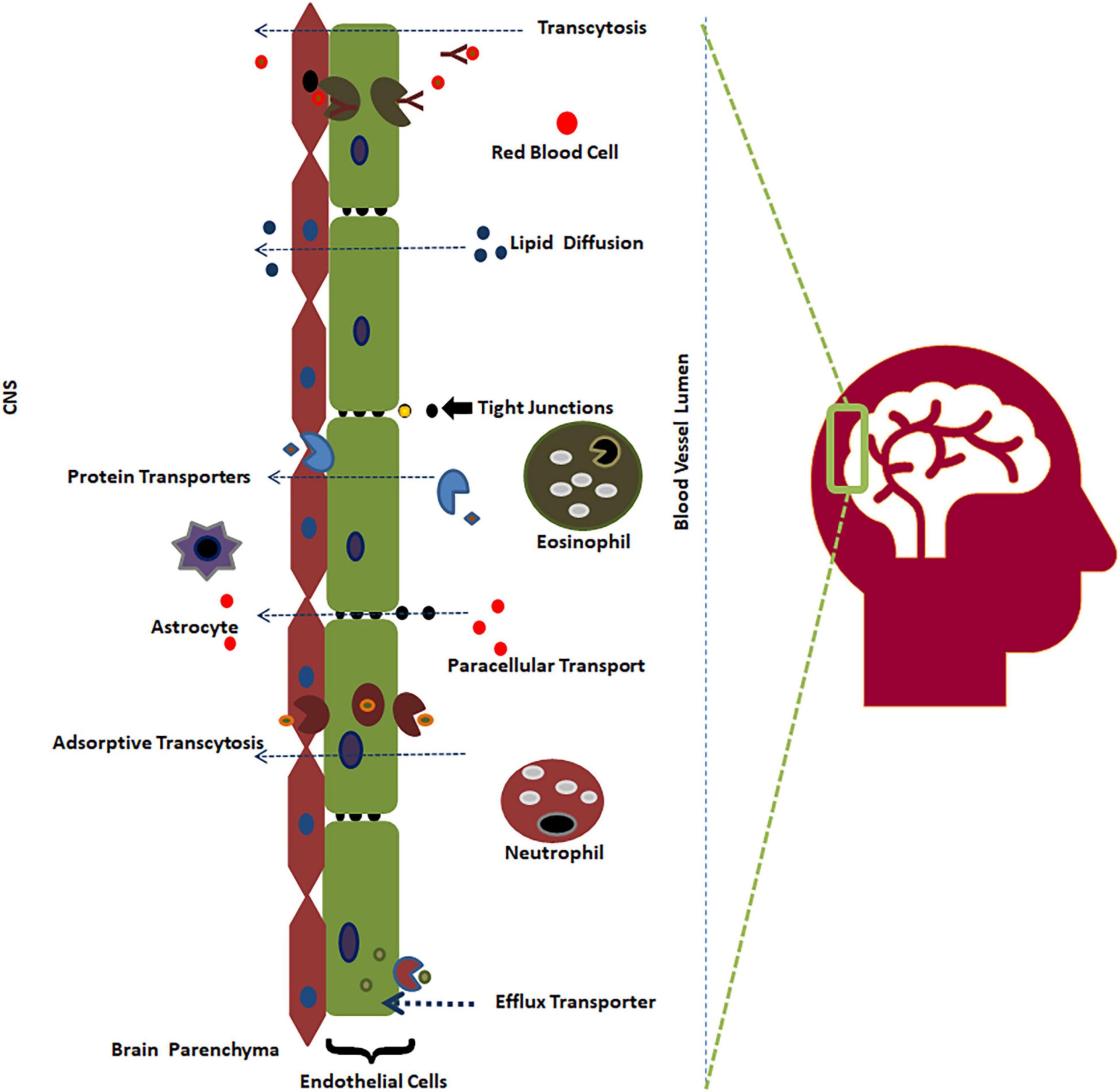
Refining Clinical Outcome Assessments (COAs) is essential for rare-disease teams aiming to capture subtle yet meaningful changes in patient health. As the pharmaceutical industry increasingly focuses on patient-centric approaches, the ability to measure these changes accurately becomes critical. Traditional COAs may not adequately reflect the unique experiences of patients with rare diseases, leading to potential gaps in data that could affect regulatory approvals and market access.
In this context, teams must prioritize the development and validation of COAs that are sensitive to the nuances of rare diseases. This involves engaging with patients and stakeholders to ensure the assessments resonate with their experiences and challenges. By doing so, companies can not only enhance the quality of their clinical data but also demonstrate a commitment to addressing the specific needs of this underserved patient population.
The implications are significant: improved COAs can lead to better-informed decision-making in drug development, ultimately resulting in therapies that are more aligned with patient needs. This alignment is crucial for gaining regulatory approval and achieving successful market entry, thereby fostering a more effective response to the challenges posed by rare diseases.
Get started today with Solo access →