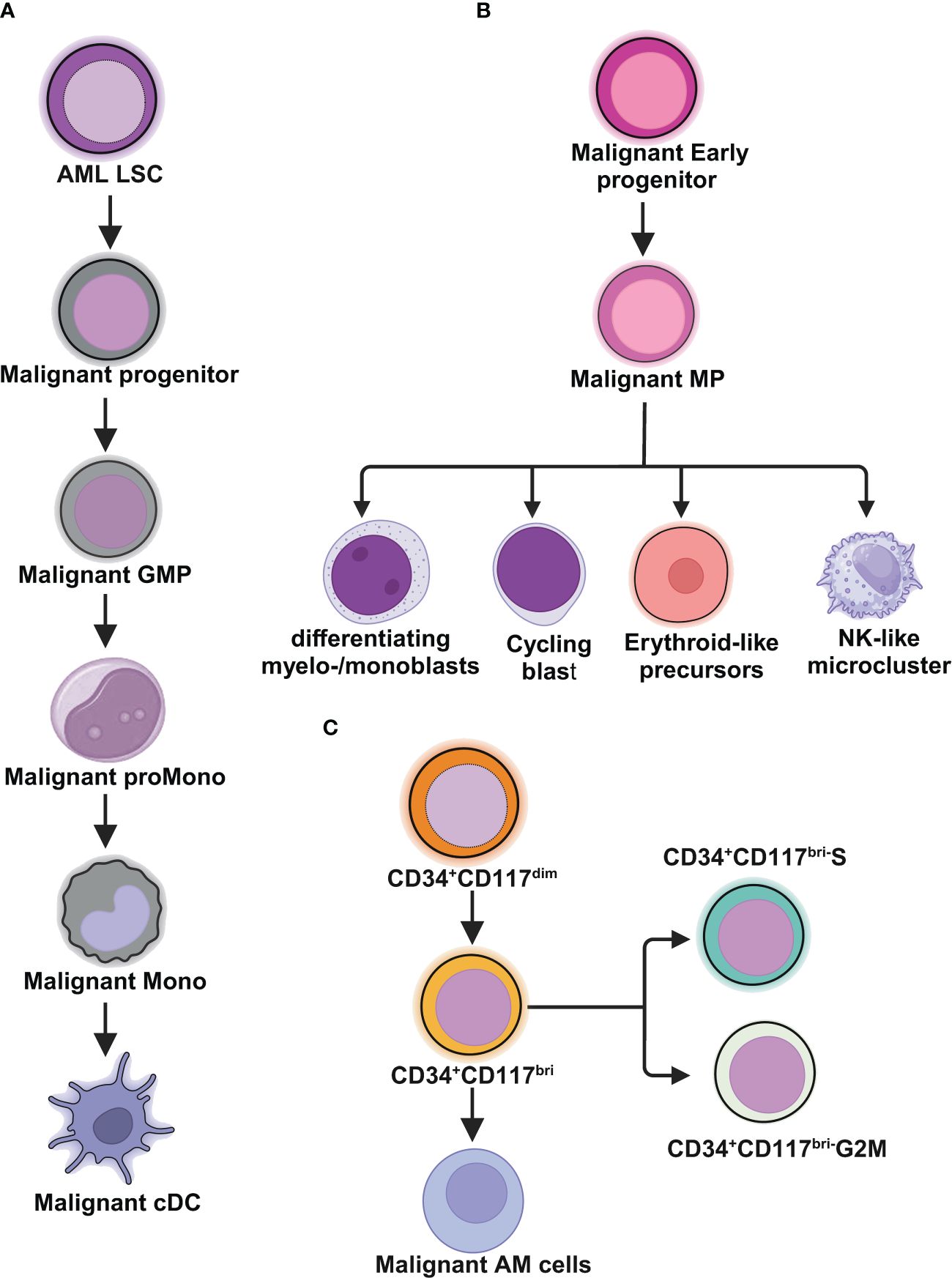
During the recent American Society of Hematology (ASH) meeting, groundbreaking results were presented for a novel therapy targeting acute myeloid leukemia (AML), showcasing significant efficacy in a patient population previously deemed difficult to treat. This development marks a pivotal moment in the ongoing battle against aggressive hematological malignancies, as the drug demonstrated a robust response rate that could alter treatment paradigms.
The implications of these findings extend beyond clinical efficacy; they signal a potential shift in the regulatory landscape as more innovative therapies enter the pipeline. As pharmaceutical companies and regulatory bodies closely monitor these advancements, the focus will likely intensify on expedited approval processes and real-world evidence to support the integration of such therapies into standard care. Industry stakeholders must prepare for the ensuing discussions on reimbursement and access, ensuring that these promising treatments can reach the patients who need them most.
Start your 7-day trial and see what the database can do →