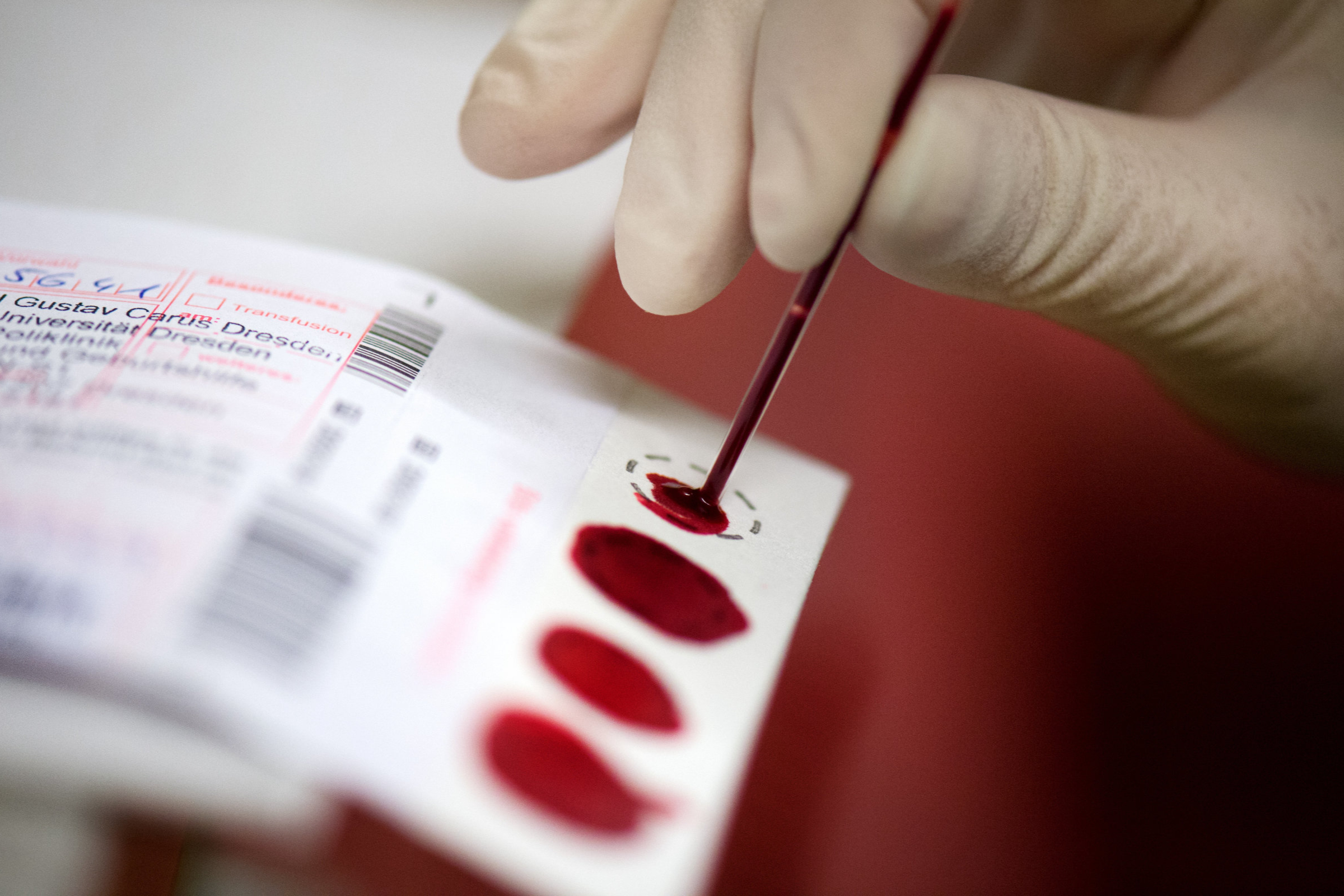
HHS Secretary Robert F. Kennedy Jr. has officially added two new conditions to the federal newborn screening list, a move that marks a significant shift in policy following the previous administration’s stagnation on this critical health initiative. This decision comes after the termination of the advisory committee that previously guided the screening process, raising questions about the future of disease additions and the overall framework for newborn health assessments.
The implications of this development are profound for stakeholders in the pharmaceutical and healthcare industries. With the addition of these conditions, there is potential for increased demand for diagnostic tests and treatments, which could drive innovation and investment in related sectors. However, the uncertainty surrounding the process for incorporating additional diseases into the screening list may create challenges for manufacturers and regulators alike, necessitating close monitoring of policy developments and their impact on market dynamics.
Use the database as your supply chain compass →