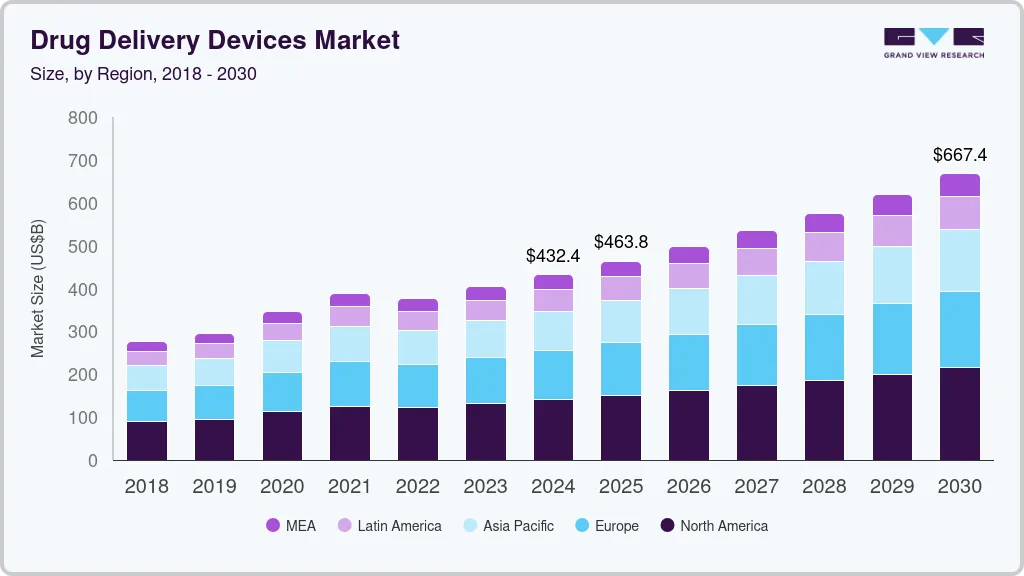
China’s National Medical Products Administration (NMPA) has granted approval for Sanofi’s Tzield, marking a significant milestone as it becomes the first disease-modifying therapy for autoimmune type 1 diabetes (T1D) in the region. This approval not only underscores the growing recognition of innovative treatment options for chronic conditions but also highlights the evolving landscape of diabetes management in China, where the prevalence of T1D is on the rise.
The introduction of Tzield is poised to reshape therapeutic strategies for healthcare providers and patients alike, offering a new avenue for disease modification rather than mere symptom management. As regulatory bodies increasingly prioritize advanced therapies, this development may prompt other pharmaceutical companies to accelerate their research and development efforts in the diabetes sector, potentially leading to a broader array of treatment options in the near future.
Get started today with Solo access →