In a new study published in Cell Reports titled, “9orf72 in myeloid cells prevents an inflammatory response to microbial glycogen,” researchers from Case Western Reserve University have identified a link between gut bacteria and the deterioration of the brain in Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia (FTD). Results identified bacterial sugars that cause neurodegenerative immune responses, providing a mechanism for therapeutic intervention.
The human gastrointestinal tract is a significant source of neuromodulatory factors and represents the largest site of microbial–host interactions, with an estimated 100 trillion microorganisms interfaced by 70%–80% of the body’s immune cells. Disruption to the microbe-immune-brain axis may contribute to neurodegeneration risk. However, how the gut microbiome is altered in patients with ALS remains poorly understood.
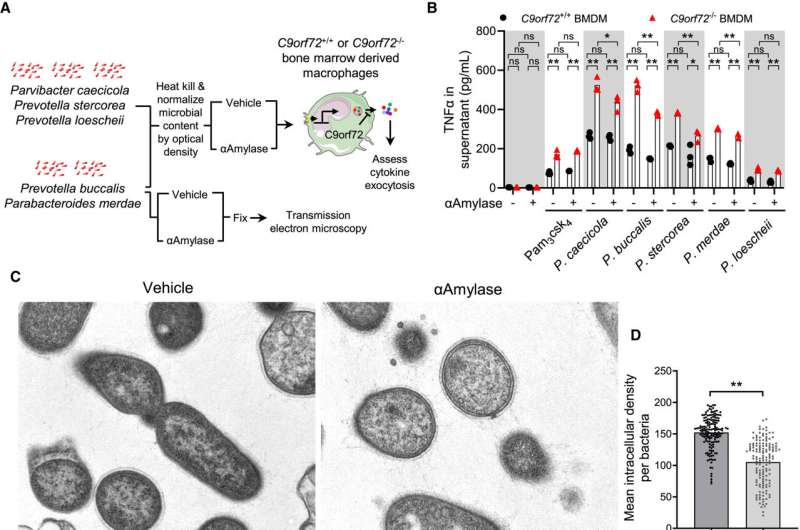
“We found that harmful gut bacteria produce inflammatory forms of glycogen, and that these bacterial sugars trigger immune responses that damage the brain,” said Aaron Burberry, PhD, assistant professor in the department of pathology at the Case Western Reserve School of Medicine and co-corresponding author of the study.
FTD affects the brain’s frontal and temporal lobes, causing changes in personality, behavior, and language, while ALS primarily targets motor neurons, leading to muscle weakness and paralysis. Although the causes of ALS and FTD are largely unknown, researchers have explored the roles of genetics, environmental factors, brain injuries, and diet in neurodegeneration.
The authors reported that 70% of the 23 ALS/FTD patients examined had dangerous glycogen levels, compared to only one-third of patients without these brain diseases. This finding highlights a potential biomarker for identifying at-risk individuals.
The study has immediate implications for patient care by identifying new targets for treating ALS and FTD, while providing biomarkers to identify patients who might benefit from gut-targeted therapies. New treatments could potentially break down harmful sugars in the gut, paving the way for drugs that target the connection between the digestive system and the brain.
Alex Rodriguez-Palacios, DVM, PhD, assistant professor in the Digestive Health Research Institute at the School of Medicine and co-corresponding author of the study, noted that reducing harmful sugars “improved brain health and extended lifespan.”
This discovery is particularly significant for C90RF72 mutation carriers, the most common genetic cause of ALS and FTD, as it elucidates why some individuals with the mutation develop the diseases while others do not, identifying gut bacteria as a key environmental trigger.
The research was facilitated by Case Western Reserve’s unique ability to conduct studies using germ-free mouse models, allowing for large-scale microbiological studies to understand the complex communication between the gut and brain.
Burberry indicated that the team will conduct larger studies surveying gut microbiome communities in ALS/FTD patients before and after disease onset to understand the timing and reasons for harmful microbial glycogen production. He emphasized that clinical trials to determine whether glycogen degradation could slow disease progression in ALS/FTD patients could begin within a year.
Use the database as your supply chain compass →