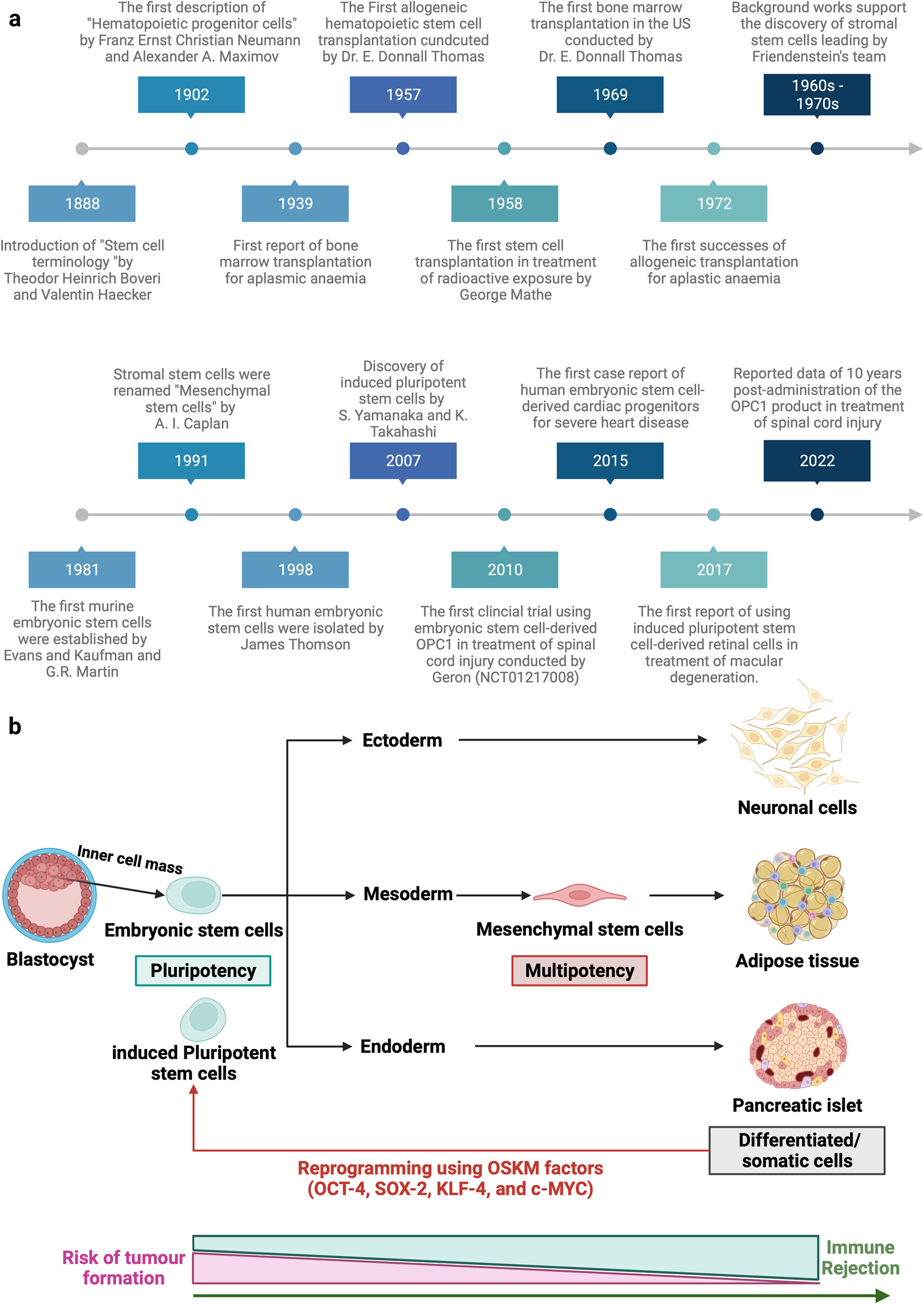
Universities must do more to prepare engineering students for the manufacturing of cell and gene therapies, according to a new analysis indicating that few degree-level training courses cover the necessary specialized skills. The author, Andy Tay, PhD, from the National University of Singapore, highlights that most programs still emphasize large molecule drug production, neglecting the unique requirements of cell and gene therapy manufacturing.
Tay explains that the production of cell, tissue, and gene therapy products (CTGTP) differs significantly from traditional biopharmaceutical manufacturing, where cells serve as hosts for therapeutic antibodies. In CTGTP, cells themselves are the medicine, making the processes of isolation, expansion, and quality control critical. The potential for error is minimal, as these cells are administered directly to patients.
The analysis reveals that training in cell and gene therapy production at top-tier institutions is inconsistent. Tay notes that many leading English-speaking universities provide inadequate preparation for undergraduates, particularly in workflow design, which is increasingly important as automation in cell manufacturing grows. He advocates for more courses that focus on these modern skills, which surpass traditional manual techniques.
While some universities in the U.K., Singapore, and the U.S. have established partnerships with drug firms to enhance training through internships, Tay emphasizes the need for broader industry collaboration. He suggests that universities should revamp their curricula to include specialized modules on CTGTP and foster connections with local biotechnology firms for practical learning experiences. Tay’s own program exemplifies this approach, incorporating visits to leading manufacturing facilities to expose students to cutting-edge technologies.
However, the feasibility of large-scale internship programs may be limited by the specialized and controlled nature of cell therapy manufacturing facilities. Tay warns that some companies might be reluctant to host multiple interns, restricting training opportunities. As a solution, he proposes the use of virtual technology to simulate commercial production environments, which could offer an innovative educational alternative in an increasingly digital learning landscape.
Start your 7-day trial and see what the database can do →