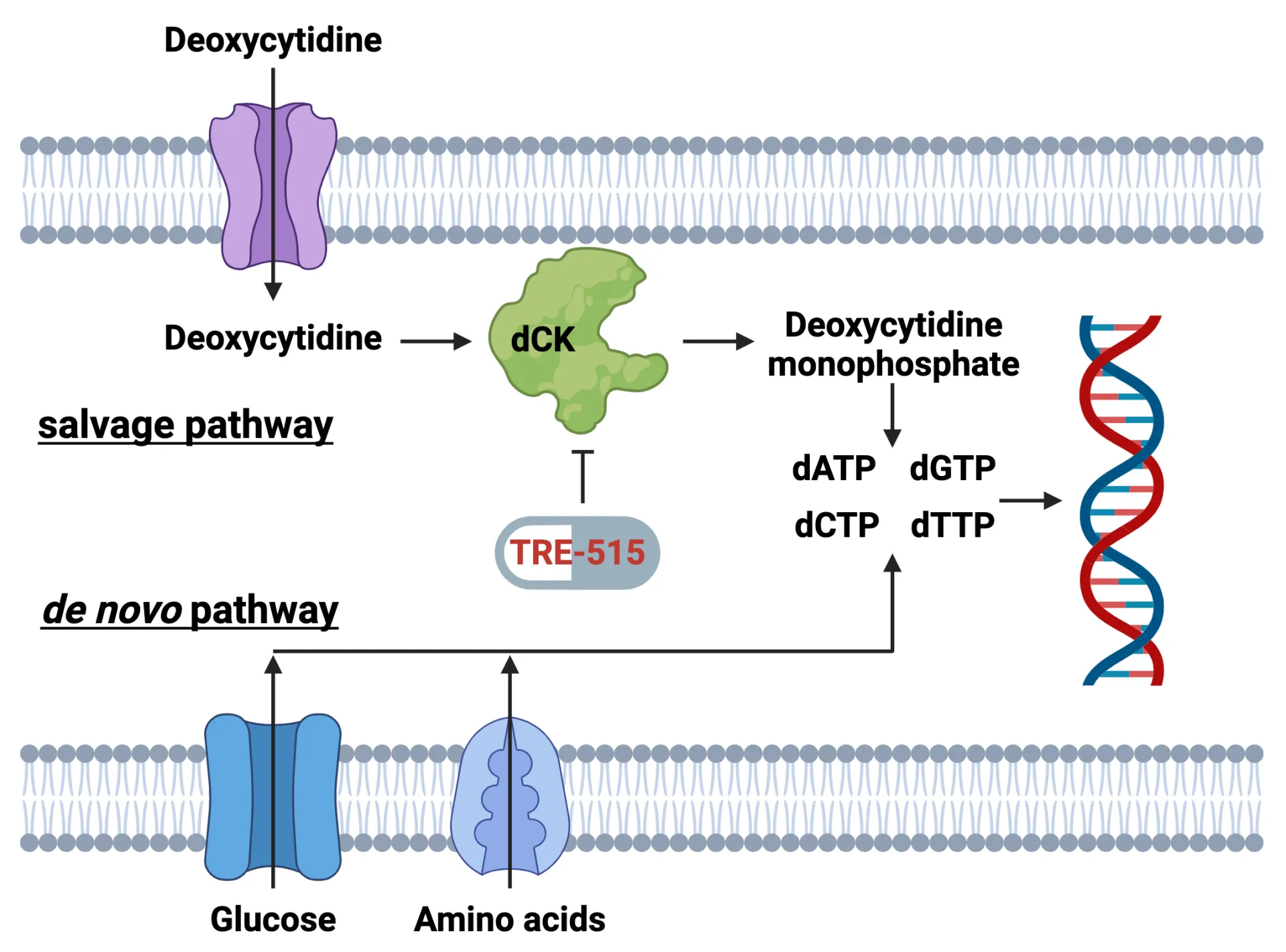
Trethera Corporation has successfully completed patient enrollment in its Phase 1 dose escalation trial for TRE-515, a novel treatment targeting advanced solid tumors. Patients have tolerated escalating doses of TRE-515, ranging from 40 mg to 1,440 mg per day, without experiencing dose-limiting toxicities. This trial is pivotal as TRE-515 inhibits deoxycytidine kinase (dCK), an enzyme essential for the nucleoside salvage pathway, which plays a critical role in the growth of cancer and autoimmune cells.
Dr. Ken Schultz, Trethera’s CEO and CMO, emphasized that this milestone positions TRE-515 as a potential first-to-market dCK inhibitor, with plans to expand clinical research in 2026, following the FDA Fast Track designation for prostate cancer. Early evidence of antitumor activity has been noted, with patients in the initial cohorts showing clinical benefits, including one maintaining disease control for over 250 days, highlighting the drug’s promising safety profile.
The trial’s primary endpoints focus on assessing the safety and tolerability of TRE-515, while secondary endpoints aim to establish a recommended Phase 2 dose and evaluate pharmacokinetics and pharmacodynamics. Supported in part by an NIH grant, Trethera’s innovative approach to targeting nucleotide metabolism could significantly impact treatment outcomes for patients with cancer and autoimmune diseases.
Start your 7-day trial and see what the database can do →