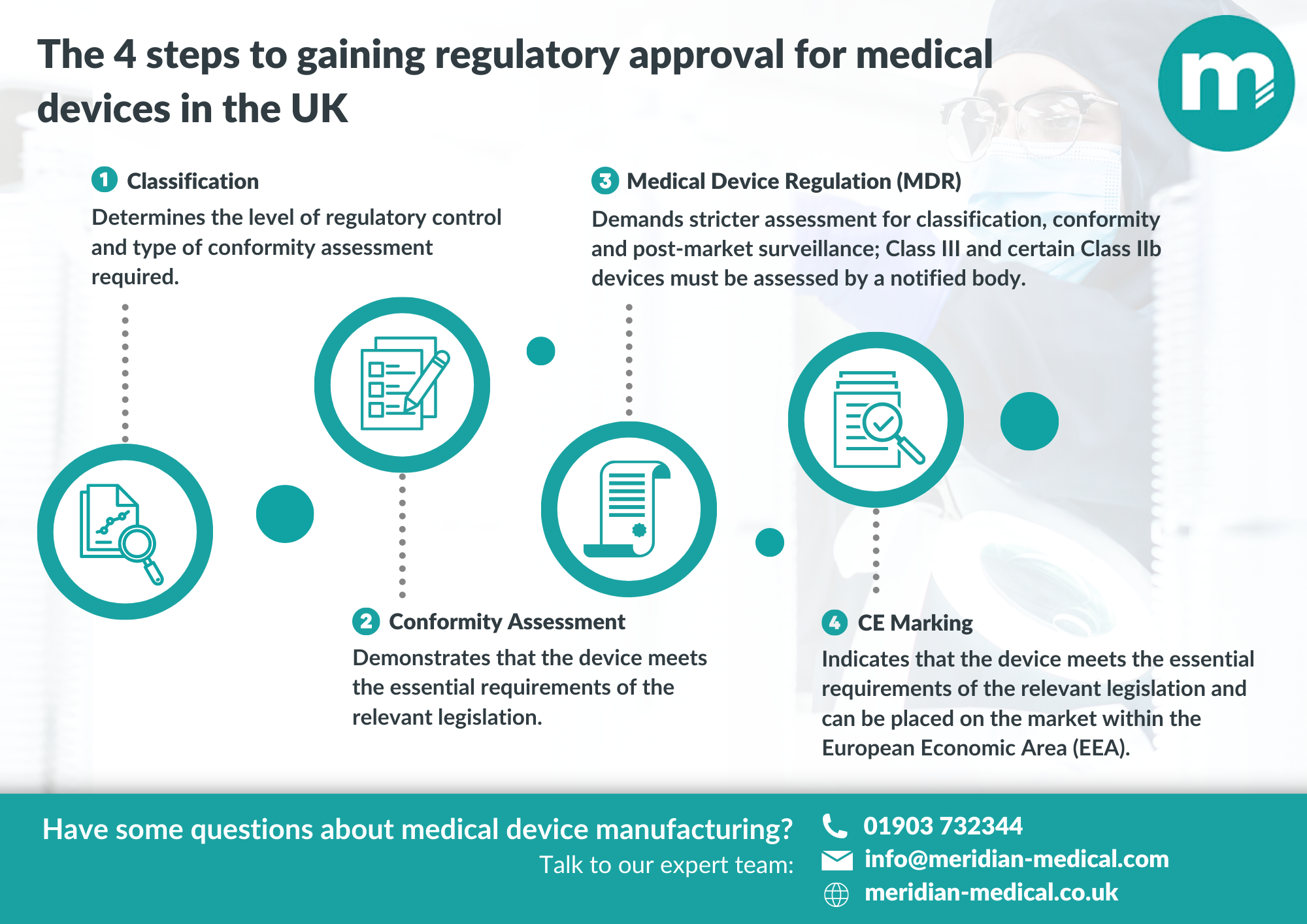
The UK government has released an updated list of organisations approved to conduct conformity assessments for medical devices, a critical step in ensuring compliance with regulatory standards. This development comes in the wake of increasing scrutiny on medical device safety and efficacy, particularly following recent global health challenges that have highlighted the importance of rigorous regulatory oversight.
With the evolving landscape of medical device regulations post-Brexit, the inclusion of these approved bodies is essential for manufacturers seeking to navigate the complexities of compliance in the UK market. The presence of multiple accredited organisations not only streamlines the assessment process but also enhances the overall quality assurance framework, fostering greater confidence among stakeholders.
For industry professionals in regulatory affairs, quality assurance, and supply chain management, understanding the implications of this guidance is paramount. It underscores the necessity for robust partnerships with approved bodies to ensure that products meet the stringent requirements set forth by UK regulations, ultimately safeguarding public health and advancing innovation in medical technology.
Get started today with Solo access →